News
Thoughts of the outgoing Past President – Axel R Pries
- Details
Towards a European Health Union: Role of the BioMed Alliance

Dear Friends and Colleagues in the BioMed Alliance,
May be, you allow me to start with a provocative question: Why are you investing time and energy into the BioMed Alliance? There is no money in it, little glory and a lot of work!
I guess that for most of us there are two main reasons, which are closely linked: A very friendly and synergistic group drives the BioMed Alliance – and that is done on the basis of a common goal:
Improve health provision throughout Europe and for all medical disciplines based on excellent health research.
That leads us without any delay to the actual efforts to establish a European Health Union. The COVID-19 pandemic has clearly demonstrated that European coordination and cooperation in health and biomedical research are vital. It was obvious that Europe has a very large potential with respect to biomedical research, innovation, transfer and medical treatment.
But the crisis has also shed a spotlight on the fact that we have a substantial problem in coordination of our efforts and in the optimal use of our resources. This is true in science and research and in the medical field – and especially at the interface between both areas, the translation. Translation is exactly the domain of the member societies of the BioMed Alliance representing over 400.000 researchers, physicians and health professionals in Europe. It is the daily business of all these societies to learn from new scientific insights, to disseminate knowledge, to foster new medical developments and to look far ahead in science based education and planning. It is also clear to all members of these societies that the medical entities they address day in day out do not respect borders or national regulations. They know that medical problems tend to be hard to fight and that success requires sustained efforts, often over decades!
It is our task in Europe to generate optimal mechanisms to deal with the fundamental international nature of diseases, science and medical treatment options in the framework of the political and organizational structure of the EU. The diversity of backgrounds, approaches and concepts in Europe provides for a very rich environment of excellence and ideas. The increasing international standing of European science reflects this. However, this positive diversity should not lead to a fragmentation of resources. The inclusive democratic control processes of the EU should not prevent sustained and coordinated initiatives in research and translation.
The Framework and Horizon programmes of the EU are by far the most significant transnational funding schemes in Europe. They bring together scientists and researchers from all countries. Nevertheless, they usually do not support the sustained efforts needed to address the most severe questions in medical science. There is always the danger of a ‘flash in the pan’ instead of the continuous development towards real solutions for large problems.
In the current discussion triggered by the CoVid Pandemic the European ‘Health Emergency Response Authority’ (HERA) was established, and is in part addressing the weaknesses described above. HERA is certainly necessary to fight future health emergencies, but it is not sufficient to unleash Europe’s potential in the field of biomedical research, translation and treatment. Its focus is on ‘Health Emergencies’ that represent only a relatively small sector of the entire medical requirements.
The BioMed Alliance has long argued for the foundation of a broader coordinating body in Europe, tentatively named ‘European Council for Health’. The European Council for Health should bring together stakeholders from civil society, industry and politics under a scientific leadership and initiate and coordinate the entire translational spectrum reaching from basic research to innovative medical solutions in prevention, diagnosis and treatment.
The European Council for Health would be a central pillar for a European Health Union by making health research and health provision in Europe more equal and more effective. Let’s work together effectively for a European Health Union by taking the responsibility for Health for all. This is a good reason to invest time and energy into the BioMed Alliance.
With my best regards
Axel R Pries
The new Code of Conduct: BioMed Alliance updates ethical framework for medical societies
- Details
The BioMed Alliance has updated its Code of Conduct, which provides a unique set of ethical principles relevant to the work of medical and research societies. Over the past years the code has been widely used and referenced as a helpful tool for the Alliance and its members to help ensure professional independence, objectivity and scientific integrity.
The Code of Conduct has now been brought up to date by a special Working Group which created a new version adapted to the current situation and setting high ethic standards and principles for medical and research societies. It was approved during the 2021 General Assembly and will continue to guide the activities of the BioMed Alliance and its members in the future.
Apply now: Additional experts needed for European Commission EXPAMED Panels
- Details

The European Commission has informed us that they are looking for additional candidates to be part of the Medical Devices Expert Panels (EXPAMED). The panels play a key role in evaluating high-risk devices and thus help to ensure the safety of medical devices and in vitro diagnostics in the EU.
The first call for experts was published in 2019, and since then the panels have started their work under the new Medical Devices Regulation and the In Vitro Diagnostics Regulation. Due to different circumstances and a large number of devices to evaluate, additional applications are welcomed in several fields and particularly in:
- Orthopaedics, traumatology, rehabilitation, rheumatology
- Endocrinology & Diabetes
- Obstetrics & Gynaecology, including reproductive medicine
- In vitro diagnostic medical devices
- Detection of arboviruses
- Detection of parasites
- Detection of haemorrhagic fever and other biosafety level 4 viruses
Interested candidates are asked to apply as soon as possible via the online application form, and you can find more information on the Commission website here or in the BioMed Alliance briefing here.
New leadership at BioMed Alliance
- Details
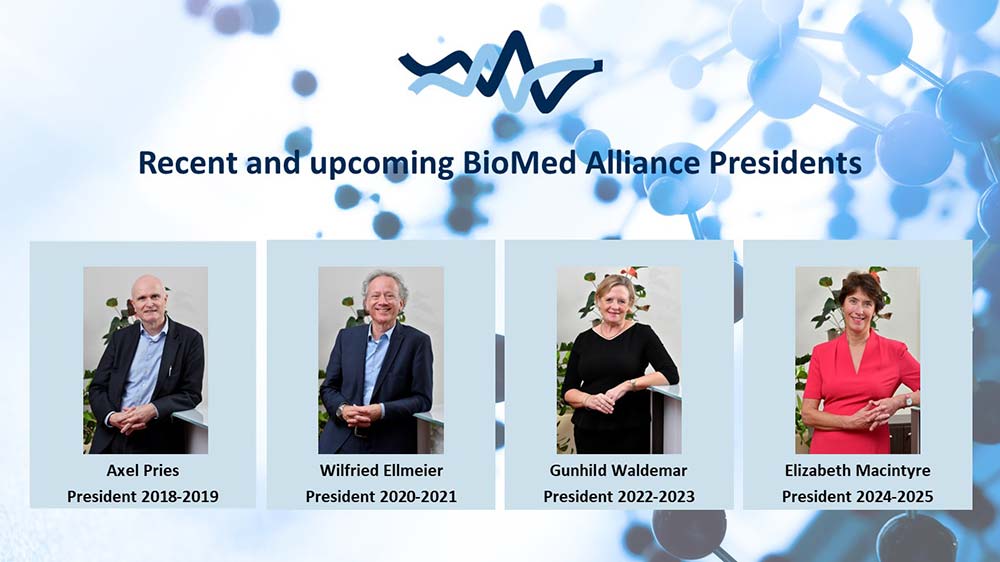
The year started well for BioMed Alliance, under the lead of its new President Prof. Gunhild Waldemar. Gunhild has been on the Board since 2017 and has been actively involved in many of our activities throughout the years. Her specialty is in neurology, and she is Professor of Clinical Neurology at Rigshospitalet, University of Copenhagen. In the coming two years she aims to help the BioMed Alliance to continue to grow, and to support its members in these turbulent times. Gunhild has taken over from Prof Wilfried Ellmeier, who has successfully been leading the BioMed Alliance over the past two years and will continue to advise as Past-President.
Prof. Elizabeth Macintyre was nominated to become the next President-Elect after Gunhild finishes her term. Elizabeth is currently President of the European Hematology Association and chairs our In Vitro Diagnostics Task Force. She has extensive experience in representing BioMed Alliance and will become President in January 2024.
Unfortunately, this also means that Prof. Axel Pries has finished his term as Past-President and will therefore leave our Board of Directors after many successful years. During his time as President-Elect, President and Past-President the BioMed Alliance grew significantly and expanded its activities. He has been a very motivated advocate for the BioMed Alliance, and he will be missed in the Board!
EFLM becomes the 33rd member of the BioMed Alliance
- Details

The European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) has now officially joined the BioMed Alliance. It is the 33rd organisation to join the BioMed Alliance, bringing important expertise related to the fields of clinical chemistry and laboratory medicine. We would like to warmly welcome them to our growing organisation.
EFLM connects National Societies of Clinical Chemistry and Laboratory Medicine and creates a platform for all European “Specialists in Laboratory Medicine”. EFLM provides European leadership in Clinical Chemistry and Laboratory Medicine to national professional societies, the diagnostic industry and to governmental and non-governmental organisations in order to serve the public interest in health care. EFLM represents the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) in Europe.
Event 12 September: Brexit: the European Parliament’s role in prioritising patients, public health and health security across Europe
- Details

On 12 September, the BioMed Alliance is joining a number of stakeholders for an event on Brexit and the European Parliament’s role in prioritising patients, public health and health security across Europe. At a critical time when there is a very strong risk of a no-deal scenario, the big questions concerning Brexit for patients across the whole EU remain unanswered. This event will focus on explaining to new MEPS the challenges of safeguarding public health and health security. It will also highlight what the European Parliament can do to follow up on its resolution from 14 March 2018 to ensure that the new European Commission is aware of the challenges and is acting to mitigate the risks.
DRAFT AGENDA
| Time | Speaker and Subject |
| 10:00-10:05 | Introduction and welcome |
| 10:05-10:20 | Usman Khan, Executive Director European Patient’s Forum - key concerns for European patients |
| 10:20-10:35 | Professor George Griffin, President, Federation of European Academies of Medicine– safeguarding European medical research post Brexit |
| 10:35-10:50 | Fiona Godfrey, Secretary General, European Public Health Alliance, preserving public health |
| 10:50-11:05 | David Boyd, Astrazeneca, access to and safety of medicines and medical devices |
| 11:05-11:20 | Pascal Garel, Secretary General, European Hospital and Healthcare Federation, challenges facing European hospitals |
| 11:20-11:40 | Question and Answers |
| 11:40-11:50 | Concluding remarks |
The event takes place on Thursday 12 September from 10.00-12.00 in the European Parliament, Room A3G2. Registration: https://forms.gle/6HWVZ7wBGpfQk2Wt7
For more information, read our Briefing on the consequences of Brexit on health and health research here: Download
Medical societies unite for high quality CME
- Details
Medical societies from different disciplines cooperate within the Biomedical Alliance in Europe (BioMed Alliance) to work towards becoming a strong common voice for high-quality Continuing Medical Education (CME) in Europe. Medical societies play a key role in the provision of high-quality education to medical professionals e.g. in the form of congresses, e-learning modules, guidelines, journals and other scientific publications.
The BioMed Alliance taskforce on the future of CME has produced an article raising awareness on the importance of unbiased and qualitative medical education. The article was written by a number of leading academics and is published ahead of print by the American Journal of Medicine on PubMed. The aim of the article is to reflect on the role of industry in CME Provision and to identify the balance that is ultimately right for patients.
A CME Experts Standing Committee was also recently established to allow for continued discussions and cooperation on medical education. The Committee held its first formal meeting on 17 April and consists of experts in medical education from BioMed Alliance medical societies. The CME Experts Committee aims to foster cooperation between medical societies and explores potential actions to guarantee excellence of educational activities. It will work together with different stakeholders to set standards on how scientific societies plan and deliver educational programmes to achieve high quality medical education for the benefit of its members with an ultimate focus on better patient care.