News
Update March 2023
- Details
Take a look at the March 2023 Update here, for information about BioMed Alliance activities and news at EU level. In this edition you will find more information about the dates of our General Assembly, our new statement on the proposal for an opt-out approach in the EHDS, additional information on our upcoming Spring Meeting, the new BeWell newsletter, the next CORE-MD webinar, the recent IMDRF conference, a call for transnational funding and Members' news.
BioMed Alliance shares reaction to opt-out model proposed in EHDS negotiations
- Details

Considering the ongoing political discussions, the BioMed Alliance has published a new statement on suggestions to introduce an opt-out model in the European Health Data Space (EHDS) proposal. BioMed Alliance believes that an opt-out model could be an appropriate way forward, if certain conditions are fulfilled to ensure it does not become a barrier to life-saving health research.
The discussions on the European Health Data Space (EHDS) proposal are continuing and there are diverging views in the Parliament on the model through which the secondary use of health data will be allowed. As a compromise, the ENVI-LIBE rapporteurs have suggested to introduce an opt-out model, where citizens can indicate that they would like to exclude the re-use of their health data from EHDS. While we believe the original Commission proposal already put in place the necessary safeguards, we do think the opt-out model could be an appropriate compromise considering the political situation, if the necessary provisions are in place to make sure it is workable in practice. It is e.g. essential that the model is implemented in a harmonised way across the EU, includes a differentiated approach for different types of data, is accompanied with sufficient information provision to EU citizens on the benefits of sharing their data and the safeguards in place to protect their privacy and if there are further elaborations on ethical principles.
Read more here
Update February 2023
- Details
Read our February Update to get more information about new developments at EU level and our recent activities. In this Update you will find more information about our new survey on the reduced availability of medical devices, our proposed EHDS amendments, our contributions to a recent MDCG meeting, an update on the Coalition for Reducing Bureaucracy in Clinical Trials, the next CORE-MD webinar and an upcoming stakeholder conference of the IMDRF.
BioMed Alliance launches new survey on reduced availability medical devices
- Details
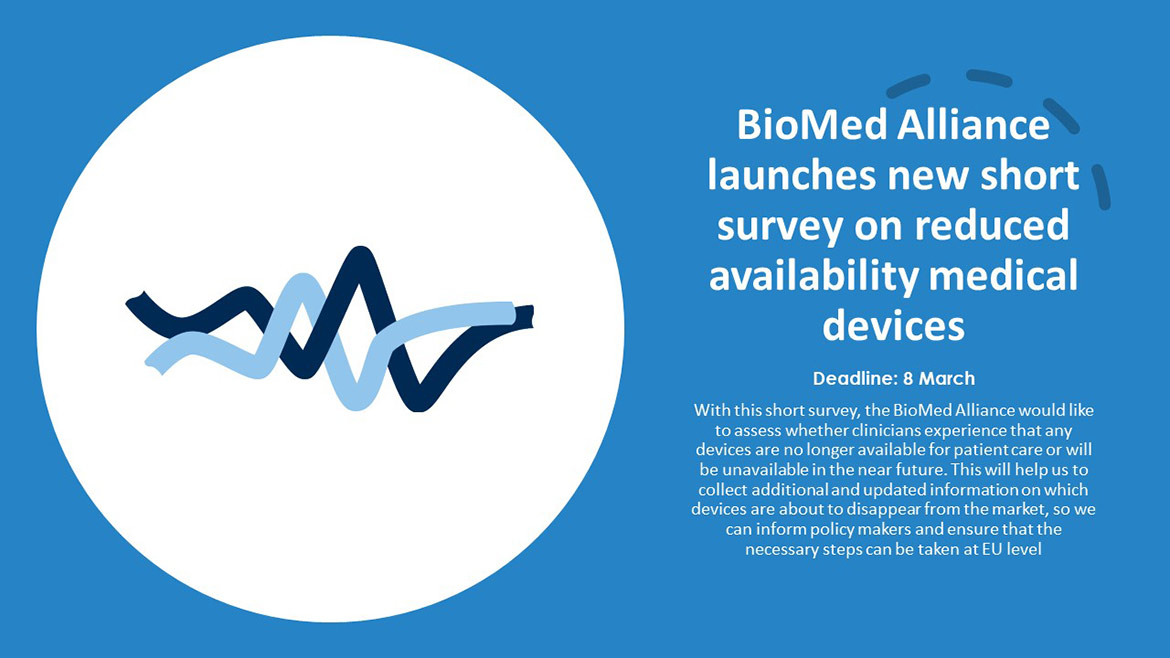
BioMed Alliance has launched a new survey on current/upcoming shortages of medical devices. With this short survey, the BioMed Alliance would like to assess whether clinicians experience that any devices are no longer available for patient care or will be unavailable in the near future. This will help us to collect additional and updated information on which devices are about to disappear from the market, so we can inform policy makers and ensure that the necessary steps can be taken at EU level. The deadline for this survey is 8 March.
This survey follows our earlier survey on clinicians' experiences with the availability of devices and will allow us to collect more elaborate and updated data. We conducted our previous survey in 2022 and it showed that clinicians already experience issues with the availability of different medical devices under the new Medical Devices Regulation (MDR) and particularly with Orphan Devices. This limited availability can have consequences for the level of patient care and the BioMed Alliance has thus launched a series of advocacy actions leading to the publication of the draft amendment to MDR transition times in January 2023.
BioMed Alliance suggests amendments to the EHDS Proposal
- Details

The 36 medical and research societies part of the Biomedical Alliance in Europe have suggested amendments that could be considered in the legislative process of the European Commission Proposal for a European Health Data Space.
The European Health Data Space (EHDS) could have a transformative effect on the healthcare and research sectors by facilitating health data sharing and use for healthcare (primary) and policy making and research (secondary) purposes. The BioMed Alliance welcomes the intention to reduce barriers to data sharing and to ensure that patients, healthcare professionals and researchers have better access to data.
After extensive discussions with researchers, healthcare professionals and policy experts in its Health Data Taskforce, the Alliance suggests several amendments that could improve the implementation of EHDS and make sure it can have a concrete positive impact on the healthcare and research sectors and ultimately on the life of patients. The document also includes case studies of health data sharing that were collected my medical societies from different disciplines.
Read the amendment document here and the adapted version according to the draft ENVI-LIBE Report from 10 February here.
BioMed Alliance welcomes 3 new members
- Details

The BioMed Alliance has welcomed 3 new member organisations this year. We look forward to working with the European Society for Blood and Marrow Transplantation (EBMT) the European Society of Paediatric Endocrinology (ESPE) and the Association for European Paediatric and Congenital Cardiology (AEPC). We are glad that these societies have decided to join us and we are excited to work with them on our growing number of activities.
About EBMT
The European Society for Blood and Marrow Transplantation (EBMT) is a collaborative peer network of professionals working in centres and as individuals in the fields of clinical bone marrow transplantation, gene therapy and cellular therapy. The EBMT has more than 6,000 members in over 70 countries.
About ESPE
The European Society for Paediatric Endocrinology (ESPE) is an international organisation aiming to improve clinical care of children and adolescents with endocrine conditions, including diabetes, through research and education.
About AEPC
The Association for European Paediatric and Congenital Cardiology (AEPC) and its Working Groups aim to enhance collaboration amongst members for scientific research and professional development and to maintain high standards of professional practice. The Members of AEPC are paediatric cardiologists and other specialists working in this field and its related disciplines originating from 32 countries in Europe, and there are also members from other parts of the world.
BioMed Alliance survey on the availability of essential medical devices
- Details
Reports from clinicians, the European Commission and manufacturers have shown that there are issues around the availability of medical devices coinciding with the implementation of the Medical Devices Regulation. Many devices are at risk of being taken of the market with serious consequences for the provision of healthcare in Europe, and therefore the European Commission has recently announced that it will soon propose new measures to address this pressing issue.
Leading up to this, BioMed Alliance published an important survey on the availability of medical devices in August 2022. It assessed if clinicians experience that any devices that they normally use are no longer available on the market. The survey was developed by BioMed Alliance in cooperation with ESC and EFORT and was available until the end of September. We received 314 replies and the survey results show that clinicians are increasingly concerned about the fact that a rising number of essential devices is no longer available for use in medical care due to various reasons, and that this affects the quality of care for patients. Particularly devices in odd sizes or intended for paediatric or orphan indications were no longer available. Often manufacturers provided MDR related reasons for the non-availabilities, including lengthy certification procedures, high costs of putting the device on the market (particularly an issue for devices intended for small patient groups) and a lack of notified bodies.
BioMed Alliance used the survey results in its advocacy efforts to make sure the issue is addressed at EU level. We have discussed the issue in our Regulatory Affairs Committee, met with permanent representations, stakeholders and industry representatives and presented our survey results in the European Commission's Medical Devices Coordination Group Meetings.
Read the survey report here.
EFLM becomes the 33rd member of the BioMed Alliance
- Details

The European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) has now officially joined the BioMed Alliance. It is the 33rd organisation to join the BioMed Alliance, bringing important expertise related to the fields of clinical chemistry and laboratory medicine. We would like to warmly welcome them to our growing organisation.
EFLM connects National Societies of Clinical Chemistry and Laboratory Medicine and creates a platform for all European “Specialists in Laboratory Medicine”. EFLM provides European leadership in Clinical Chemistry and Laboratory Medicine to national professional societies, the diagnostic industry and to governmental and non-governmental organisations in order to serve the public interest in health care. EFLM represents the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) in Europe.
Event 12 September: Brexit: the European Parliament’s role in prioritising patients, public health and health security across Europe
- Details

On 12 September, the BioMed Alliance is joining a number of stakeholders for an event on Brexit and the European Parliament’s role in prioritising patients, public health and health security across Europe. At a critical time when there is a very strong risk of a no-deal scenario, the big questions concerning Brexit for patients across the whole EU remain unanswered. This event will focus on explaining to new MEPS the challenges of safeguarding public health and health security. It will also highlight what the European Parliament can do to follow up on its resolution from 14 March 2018 to ensure that the new European Commission is aware of the challenges and is acting to mitigate the risks.
DRAFT AGENDA
| Time | Speaker and Subject |
| 10:00-10:05 | Introduction and welcome |
| 10:05-10:20 | Usman Khan, Executive Director European Patient’s Forum - key concerns for European patients |
| 10:20-10:35 | Professor George Griffin, President, Federation of European Academies of Medicine– safeguarding European medical research post Brexit |
| 10:35-10:50 | Fiona Godfrey, Secretary General, European Public Health Alliance, preserving public health |
| 10:50-11:05 | David Boyd, Astrazeneca, access to and safety of medicines and medical devices |
| 11:05-11:20 | Pascal Garel, Secretary General, European Hospital and Healthcare Federation, challenges facing European hospitals |
| 11:20-11:40 | Question and Answers |
| 11:40-11:50 | Concluding remarks |
The event takes place on Thursday 12 September from 10.00-12.00 in the European Parliament, Room A3G2. Registration: https://forms.gle/6HWVZ7wBGpfQk2Wt7
For more information, read our Briefing on the consequences of Brexit on health and health research here: Download
Medical societies unite for high quality CME
- Details
Medical societies from different disciplines cooperate within the Biomedical Alliance in Europe (BioMed Alliance) to work towards becoming a strong common voice for high-quality Continuing Medical Education (CME) in Europe. Medical societies play a key role in the provision of high-quality education to medical professionals e.g. in the form of congresses, e-learning modules, guidelines, journals and other scientific publications.
The BioMed Alliance taskforce on the future of CME has produced an article raising awareness on the importance of unbiased and qualitative medical education. The article was written by a number of leading academics and is published ahead of print by the American Journal of Medicine on PubMed. The aim of the article is to reflect on the role of industry in CME Provision and to identify the balance that is ultimately right for patients.
A CME Experts Standing Committee was also recently established to allow for continued discussions and cooperation on medical education. The Committee held its first formal meeting on 17 April and consists of experts in medical education from BioMed Alliance medical societies. The CME Experts Committee aims to foster cooperation between medical societies and explores potential actions to guarantee excellence of educational activities. It will work together with different stakeholders to set standards on how scientific societies plan and deliver educational programmes to achieve high quality medical education for the benefit of its members with an ultimate focus on better patient care.