
Description
The Task Force on In Vitro diagnostics was established based on decisions in the BioMed Alliance Taskforce on Regulatory Affairs and Medical Devices.
A new EU regulation on In Vitro Diagnostics will come into effect in 2022 and will have a major impact on the evaluation and approval process of IVDs. In preparation for this, and to facilitate member society cooperation on the regulatory aspects of in vitro diagnostics, this task force composed of IVD experts was established in 2019.
Chair
This task force is chaired by Professor Michael Neumaier.
Highlights
-
Statement: Urgent action needed to prevent widespread shortage of diagnostic tests
In a statement from January 2024, BioMed Alliance highlights its increasing concerns about approaching widespread issues with the availability of In Vitro Diagnostic Devices (IVDs) in Europe. Persisting problems with the implementation of the In Vitro Diagnostics Regulation (IVDR), insufficient certificates issued, the failure to deliver the EUDAMED database and general unpreparedness of the sector could lead to the disappearance from the market of a large number of essential IVDs in the near future and related loss of international competitivity compared to other jurisdictions. The document calls for a mix of short-term solutions (including an extension of the transition period) and more longer-term reforms of the system to ensure pressing challenges are addressed.
-
BioMed Alliance article on the implications of IVDR for innovation in diagnostics in HemaSphere
The BioMed Alliance In Vitro Diagnostics (IVD) Task Force has written an article on the Critical Implications of the In Vitro Diagnostics Regulation (IVDR) for Innovation in Diagnostics, which was published in June 2022 in HemaSphere and is available here.
The article addresses different elements around the IVDR implementation and its consequences for innovation and the work of laboratories, including for the development and use of in-house devices. It builds on the conclusions from our recent survey addressing laboratory preparedness for the IVDR and provides recommendations on important challenges to be addressed and for the way forward.
-
Useful documents for the IVDR requirements
In-house IVDs (IH-IVDs or often called LDTs) are exempt from most provisions of Regulation (EU) 2017/746 (In Vitro Diagnostic Medical Devices Regulation, IVDR) provided they meet the conditions set out in Article 5(5) of the IVDR.
The Association of the Scientific Medical Societies in Germany (AWMF) ad hoc commission provides documents as recommendations and assistance for the following IVDR requirements:
- 5 (5 f): Public statement confirming compliance with general safety and performance requirements
- 5 (5): Determination and documentation of compliance with the general safety and performance requirements listed in Appendix I.
- Detailed checklist in which each individual point of Annex I of the IVDR is addressed and evaluated. The 2nd tab is particularly helpful for laboratories that are accredited according to ISO15189.
- Compact checklist that queries the requirements of the IVDR for the commissioning of IH-IVD in the categories reagent, reagent product, calibrator, control material, kit.
- Appendix I chap. I: IVDR compliant risk management
- Appendix I, chap. II (9): IVDR compliant performance evaluation
- Handouts for validation of virological / microbiological examination methods
-
BioMed Alliance welcomes the adoption of the European Commission’s proposal amending the IVDR transition periods
BioMed Alliance welcomes the adoption of the European Commission's Proposal to amend the transitional provisions of the IVDR following the favourable vote of the Council yesterday, 20 December 2021 and its adoption in plenary at the European Parliament last week. The adoption of the Proposal was necessary to ensure proper implementation of the IVDR and avoid potentially disastrous consequences for the diagnostic sector. As representatives of many specialties involved in innovative diagnostics, we are particularly relieved that the amended transitional provisions include those involving in-house testing.
However, work still needs to be done to provide the necessary guidance and documents enabling a safe and timely transition to the new framework:
- Compliance to the IVDR will be a major effort for diagnostic laboratories, requiring trained personnel, additional time and budget. The Commission must take this into account and put forward solutions to support laboratories in minimising the rising costs.
- Guidance documents are urgently needed in order to help diagnostic laboratories to properly conduct the transformations required from the IVDR.
- Clarification on modified use of CE-IVD and RUO (research use only) tests is necessary in order to allow labs to take appropriate steps for their safe and legitimate use under the IVDR.
We look forward to continuing our collaboration with the European Commission and to supporting the key actors in accelerating the efforts to optimise diagnostic practise according to the Joint Implementation Plan.
-
Compliance to the IVDR will be a major effort for the diagnostic laboratories
The BioMed Alliance Taskforce on IVD, in collaboration with the European Haematology Association and the European Federation of Clinical Chemistry and Laboratory Medicine, conducted a survey on the current use of different IVD tests in diagnostic laboratories.
The objective of the questionnaire was to gain insight into the current situation for medical laboratories, in particular on the degree of preparedness of medical laboratories for the IVDR implementation, and to make an accurate assessment of the potential impact that the IVDR will have on diagnostic laboratories and their test menu. In total, we received 203 answers from medical laboratories across the EU and Norway. We analysed all the responses received and elaborated a report showcasing the main results as well as some explanatory figures.
You may read the entire report with the main findings here.
-
The European Commission announced delays in the IVDR implementation
The European Commission published a legislative proposal amending the in-Vitro Diagnostics Regulation (IVDR), and more specifically amending the transitional provisions for certain in-vitro diagnostic devices and differing the application of requirement for in-house devices. The date of application of the IVDR remains on 26 May 2022 but the legislative amending act is extending several transitional provisions until 2028 depending on the scope of the devices and on a risk-based approach. Extended transitional periods are also provided for certain requirements for In-house devices. In order to clearly present you the amendments proposed by the Commission we made a short recap table that you may see below the article.
BioMed Alliance welcomes the publication of this proposal and we are working on a reaction. The legislative amending act was necessary to ensure proper IVDR implementation and to answer serious concerns of the diagnostic health community about the IVDR implementation status and the potentially disastrous consequences for diagnostic practice and patients’ safety in the European Union. However, we have some reservations regarding the provisions on In-house devices.
The next steps are that the European Parliament and the Council must adopt the proposal and the MDCG and the Commission will continue to work according to the Joint Implementation Plan. We are also carrying on our work and advocacy efforts to ensure necessary steps are taken, guidance documents will be published shortly and the Notified Body capacity will be increased.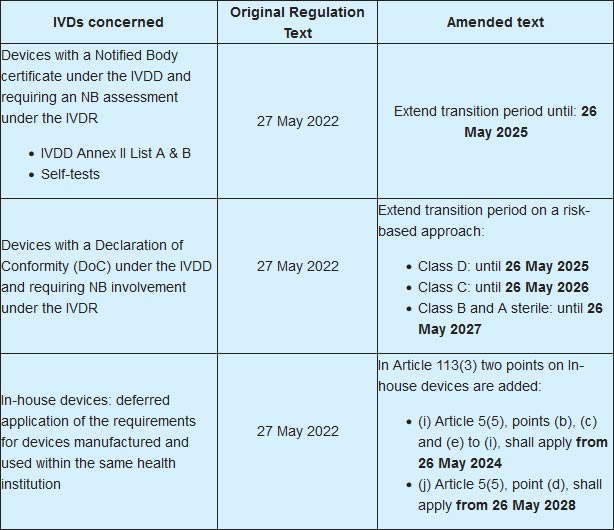
-
Statement calling for urgent actions to prevent a collapse of the diagnostic sector
In May 2021, the BioMed Alliance published a new statement highlighting a looming threat for the diagnostic sector: the slow implementation of the new In Vitro Diagnostic Regulation (IVDR), a ticking bomb for the diagnostic sector. The laboratory experts of the IVD Task Force called for immediate actions to prevent its collapse.
The application date of the new In Vitro Diagnostics Regulation is only one year away (26 May 2022) and there are widespread concerns that the implementation is not progressing quickly enough and that the diagnostic sector is not ready. The new regulation proposes stricter safeguards for tests and additional elements in the regulatory framework for IVD tests, but so far, its implementation has been slow. As a result, not all elements and guidance are in place, making it harder for the diagnostic sector to prepare. Read the statement here.
-
STOA Workshop: The need for better EU policies for health
In April, the European Parliament Panel for the Future of Science and Technology (STOA) organised a workshop on the new IVD Regulation and the need more evidence-based EU health related policies, in cooperation with the BioMed Alliance, ESC, EFLM and EHA. The meeting brought together a mix of policy makers and experts from the field to discuss challenges around the new In Vitro Diagnostics Regulation and the need for more evidence-based EU health related policies. At this occasion, the BioMed Alliance, EFLM, EHA and ESC published a press release, and following the meeting, the STOA Panel published a report on their website. For more information, the report from the STOA Panel is available here, and the BioMed Alliance press release here.
-
Statement calling to speed-up IVDR implementation
In July 2020, the BioMed Alliance published a statement calling for urgent action to ensure optimal implementation of the EU In Vitro Diagnostic devices regulation (IVDR). The statement calls on the European Commission to take into account a number of actions that should be completed as soon as possible.
The application date for the new regulation on 26 May 2022 is approaching and adaptation to the new regulatory system is time consuming and requires a concerted effort from the European Commission, Member States and stakeholders. At the moment there are many steps that still need to be taken to ensure the regulatory system will be ready to continue guaranteeing the availability of novel and high-quality diagnostic tests. Read the statement here
-
Participation MDCG Working Group on IVD
Prof. Elizabeth Macintyre (Chair of the IVD Task Force) is representing us and provides scientific input in the European Commission Medical Devices Coordination Group (MDCG) IVD Working Group. Input from our members along with solutions for a smooth implementation of the regulation will be included in the work of the EC IVD Working Group
-
Kick-off meeting
The kick-off meeting of the new Task Force on In Vitro Diagnostics took place on 12 November 2019 and brought together experts from member societies and other key players in the field. During the meeting, we discussed the new regulation with representatives of the European Commission, Industry, Notified Bodies and Medical Societies.






