News - Archive
Update February 2022
- Details
Read our February Update to get more information on our activities and the latest news at EU level. In this edition you can find more information on our statement on the war in Ukraine, our upcoming Spring Meeting & Workshop on Health Research, our new article on CME, a consultation on EU Health Data, our efforts on IVDR, and members news. Read more here.
BioMed Alliance calls for support for the population affected by the war in Ukraine
- Details

The BioMed Alliance has followed developments around the Russian army's invasion of Ukraine with great concern. We believe that the health and wellbeing of citizens must come first: lives must be protected, and citizens must continue to be able to access essential services and get the care that they need.
It is time for the European Union and the international community to stand in solidarity with the population affected by this war, and to provide support and protection to those in need of help.
In time of crisis, humanitarian health assistance, collaboration and mutual aid must prevail and the biomedical health research community can contribute and support the work of international organisations like the International Committee of the Red Cross, United Nations High Commissioner of Refugees and the World Health Organisation. Support is needed on all fronts, for example by providing funds to recognised humanitarian organisations or even by participating in the Emergency Medical Teams being deployed by WHO in the area.
We are glad to see that our members are already providing support to medical societies and clinicians in the conflict area, and additional support from the international community and relief organisations is necessary to allow healthcare professionals to provide the best care to the victims of this conflict.
The principles of international, humanitarian and human rights law need to be upheld, and we call for a swift and peaceful resolution to the conflict.

Article: Continuing Medical Education in time of crisis
- Details
The BioMed Alliance has published an article in the Journal of European CME entitled ‘Continuing Medical Education (CME) in time of crisis: How medical societies face challenges and adapt to provide unbiased CME’. The article is based on the results of two surveys on this topic conducted by the CME Experts Permanent Committee in 2019 and 2021 and evaluates the CME landscape, how medical societies develop and assess CME and how they interact with other stakeholders. It also reflects on the consequences of the COVID-19 Pandemic for the provision of CME and the challenges that are ahead. Read the article here.

Update January 2022
- Details
Take a look at our January Update to get an overview of BioMed Alliance activities and the latest news on EU health & research policy. This month you can read about the dates of our Spring Meeting & General Assembly, our statement on the EHDS, a special message from Past-President Axel Pries, the European Commission call for experts in medical devices & IVDs, our updated Statutes & Code of Conduct, a survey on Quality Indicators, an update from our IVDR taskforce and information on new health research funding calls.
Thoughts of the outgoing Past President – Axel R Pries
- Details
Towards a European Health Union: Role of the BioMed Alliance

Dear Friends and Colleagues in the BioMed Alliance,
May be, you allow me to start with a provocative question: Why are you investing time and energy into the BioMed Alliance? There is no money in it, little glory and a lot of work!
I guess that for most of us there are two main reasons, which are closely linked: A very friendly and synergistic group drives the BioMed Alliance – and that is done on the basis of a common goal:
Improve health provision throughout Europe and for all medical disciplines based on excellent health research.
That leads us without any delay to the actual efforts to establish a European Health Union. The COVID-19 pandemic has clearly demonstrated that European coordination and cooperation in health and biomedical research are vital. It was obvious that Europe has a very large potential with respect to biomedical research, innovation, transfer and medical treatment.
But the crisis has also shed a spotlight on the fact that we have a substantial problem in coordination of our efforts and in the optimal use of our resources. This is true in science and research and in the medical field – and especially at the interface between both areas, the translation. Translation is exactly the domain of the member societies of the BioMed Alliance representing over 400.000 researchers, physicians and health professionals in Europe. It is the daily business of all these societies to learn from new scientific insights, to disseminate knowledge, to foster new medical developments and to look far ahead in science based education and planning. It is also clear to all members of these societies that the medical entities they address day in day out do not respect borders or national regulations. They know that medical problems tend to be hard to fight and that success requires sustained efforts, often over decades!
It is our task in Europe to generate optimal mechanisms to deal with the fundamental international nature of diseases, science and medical treatment options in the framework of the political and organizational structure of the EU. The diversity of backgrounds, approaches and concepts in Europe provides for a very rich environment of excellence and ideas. The increasing international standing of European science reflects this. However, this positive diversity should not lead to a fragmentation of resources. The inclusive democratic control processes of the EU should not prevent sustained and coordinated initiatives in research and translation.
The Framework and Horizon programmes of the EU are by far the most significant transnational funding schemes in Europe. They bring together scientists and researchers from all countries. Nevertheless, they usually do not support the sustained efforts needed to address the most severe questions in medical science. There is always the danger of a ‘flash in the pan’ instead of the continuous development towards real solutions for large problems.
In the current discussion triggered by the CoVid Pandemic the European ‘Health Emergency Response Authority’ (HERA) was established, and is in part addressing the weaknesses described above. HERA is certainly necessary to fight future health emergencies, but it is not sufficient to unleash Europe’s potential in the field of biomedical research, translation and treatment. Its focus is on ‘Health Emergencies’ that represent only a relatively small sector of the entire medical requirements.
The BioMed Alliance has long argued for the foundation of a broader coordinating body in Europe, tentatively named ‘European Council for Health’. The European Council for Health should bring together stakeholders from civil society, industry and politics under a scientific leadership and initiate and coordinate the entire translational spectrum reaching from basic research to innovative medical solutions in prevention, diagnosis and treatment.
The European Council for Health would be a central pillar for a European Health Union by making health research and health provision in Europe more equal and more effective. Let’s work together effectively for a European Health Union by taking the responsibility for Health for all. This is a good reason to invest time and energy into the BioMed Alliance.
With my best regards
Axel R Pries
The new Code of Conduct: BioMed Alliance updates ethical framework for medical societies
- Details
The BioMed Alliance has updated its Code of Conduct, which provides a unique set of ethical principles relevant to the work of medical and research societies. Over the past years the code has been widely used and referenced as a helpful tool for the Alliance and its members to help ensure professional independence, objectivity and scientific integrity.
The Code of Conduct has now been brought up to date by a special Working Group which created a new version adapted to the current situation and setting high ethic standards and principles for medical and research societies. It was approved during the 2021 General Assembly and will continue to guide the activities of the BioMed Alliance and its members in the future.
Apply now: Additional experts needed for European Commission EXPAMED Panels
- Details

The European Commission has informed us that they are looking for additional candidates to be part of the Medical Devices Expert Panels (EXPAMED). The panels play a key role in evaluating high-risk devices and thus help to ensure the safety of medical devices and in vitro diagnostics in the EU.
The first call for experts was published in 2019, and since then the panels have started their work under the new Medical Devices Regulation and the In Vitro Diagnostics Regulation. Due to different circumstances and a large number of devices to evaluate, additional applications are welcomed in several fields and particularly in:
- Orthopaedics, traumatology, rehabilitation, rheumatology
- Endocrinology & Diabetes
- Obstetrics & Gynaecology, including reproductive medicine
- In vitro diagnostic medical devices
- Detection of arboviruses
- Detection of parasites
- Detection of haemorrhagic fever and other biosafety level 4 viruses
Interested candidates are asked to apply as soon as possible via the online application form, and you can find more information on the Commission website here or in the BioMed Alliance briefing here.
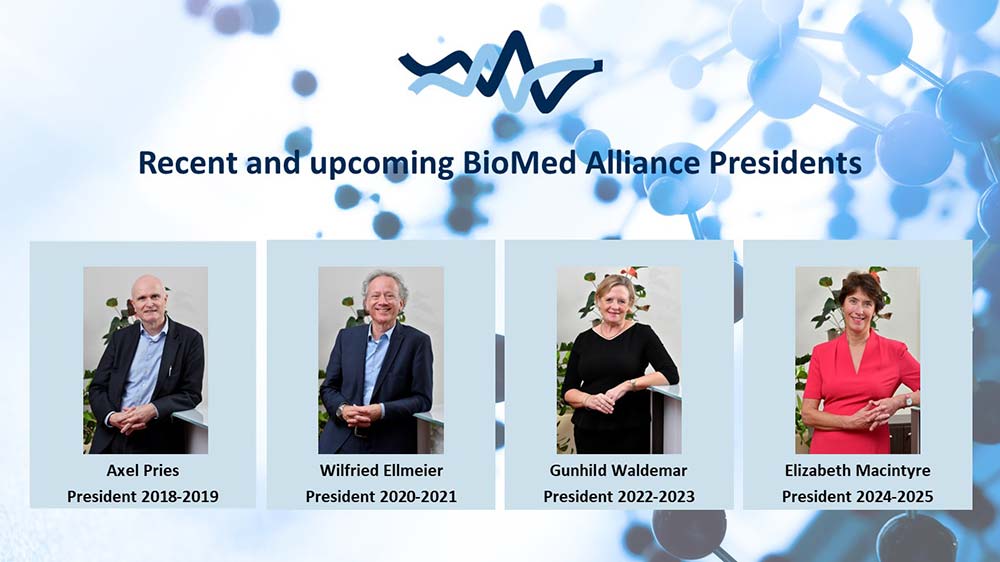
New leadership at BioMed Alliance
- Details
The year started well for BioMed Alliance, under the lead of its new President Prof. Gunhild Waldemar. Gunhild has been on the Board since 2017 and has been actively involved in many of our activities throughout the years. Her specialty is in neurology, and she is Professor of Clinical Neurology at Rigshospitalet, University of Copenhagen. In the coming two years she aims to help the BioMed Alliance to continue to grow, and to support its members in these turbulent times. Gunhild has taken over from Prof Wilfried Ellmeier, who has successfully been leading the BioMed Alliance over the past two years and will continue to advise as Past-President.
Prof. Elizabeth Macintyre was nominated to become the next President-Elect after Gunhild finishes her term. Elizabeth is currently President of the European Hematology Association and chairs our In Vitro Diagnostics Task Force. She has extensive experience in representing BioMed Alliance and will become President in January 2024.
Unfortunately, this also means that Prof. Axel Pries has finished his term as Past-President and will therefore leave our Board of Directors after many successful years. During his time as President-Elect, President and Past-President the BioMed Alliance grew significantly and expanded its activities. He has been a very motivated advocate for the BioMed Alliance, and he will be missed in the Board!
BioMed Alliance welcomes the adoption of the European Commission’s proposal amending the IVDR transition periods.
- Details
BioMed Alliance welcomes the adoption of the European Commission's Proposal to amend the transitional provisions of the IVDR following the favourable vote of the Council yesterday, 20 December 2021 and its adoption in plenary at the European Parliament last week. The adoption of the Proposal was necessary to ensure proper implementation of the IVDR and avoid potentially disastrous consequences for the diagnostic sector. As representatives of many specialties involved in innovative diagnostics, we are particularly relieved that the amended transitional provisions include those involving in-house testing.
However, work still needs to be done to provide the necessary guidance and documents enabling a safe and timely transition to the new framework:
- Compliance to the IVDR will be a major effort for diagnostic laboratories, requiring trained personnel, additional time and budget. The Commission must take this into account and put forward solutions to support laboratories in minimising the rising costs.
- Guidance documents are urgently needed in order to help diagnostic laboratories to properly conduct the transformations required from the IVDR.
- Clarification on modified use of CE-IVD and RUO (research use only) tests is necessary in order to allow labs to take appropriate steps for their safe and legitimate use under the IVDR.
We look forward to continuing our collaboration with the European Commission and to supporting the key actors in accelerating the efforts to optimise diagnostic practise according to the Joint Implementation Plan.
Update December 2021
- Details
Another year has come to an end, and it has been a busy one again for BioMed Alliance. In our special end-of-the year Update you will get an overview of this year’s key highlights and a glimpse of what is to come in 2022. Read the December Update here.
New statement: EHDS, an opportunity to unleash the power of data?
- Details

BioMed Alliance has released a new statement ahead of the upcoming European Commission legislative proposal on the European Health Data Space (EHDS). The new EHDS can play a key role in facilitating health data sharing for healthcare provision, health research and policy making if certain key aspects are taken into account.
The BioMed Alliance Task Force on Health Data Sharing, in close cooperation with the European Association of Urology (EAU) and the European Organisation for Research and Treatment of Cancer (EORTC) have therefore proposed a set of recommendations for the governance, implementation and incentivisation of adoption of the EHDS. The statement particularly highlights the need to involve a broad range of stakeholders and to align different legislative approaches with a clear and enabling EU legal framework
Read the statement here
Compliance to the IVDR will be a major effort for the diagnostic laboratories
- Details
The BioMed Alliance Taskforce on IVD, in collaboration with the European Haematology Association and the European Federation of Clinical Chemistry and Laboratory Medicine, conducted a survey on the current use of different IVD tests in diagnostic laboratories.
The objective of the questionnaire was to gain insight into the current situation for medical laboratories, in particular on the degree of preparedness of medical laboratories for the IVDR implementation, and to make an accurate assessment of the potential impact that the IVDR will have on diagnostic laboratories and their test menu. In total, we received 203 answers from medical laboratories across the EU and Norway. We analysed all the responses received and elaborated a report showcasing the main results as well as some explanatory figures.
The results to the questionnaire showed that respondents represent 25 out of 27 EU countries, with a wide range of diagnostic fields and laboratory types. Responses show notably that compliance to the IVDR will be a major effort for diagnostic laboratories, requiring trained personal, additional time and budget. It was also clear that guidance documents are urgently needed to help diagnostic laboratories to properly conduct the transformations required from the IVDR, and many respondents see the availability of appropriate guidance both as an issue/problem and a solution. According to the respondents, various forms of guidance could be used, whether as templates, practical examples, or workshops, once again requiring appropriate resources. Furthermore, it stands out from the responses that postponement of the date of application of the IVDR was greatly needed in order to ensure the continuity of high-quality diagnostic care of patients. Fortunately, this action has already been initiated in October 2021 by the European Commission. Therefore, the diagnostics labs call on the European Parliament and the Council to quickly adapt the regulation timelines and on the MDCG and the Commission to continue the work according to the Joint Implementation Plan, particularly with respect to appropriate guidance documents.
For more information, you may read the entire report on the main findings here.
Reducing Bureaucracy in Clinical Trials: the Coalition Recommendations are out!
- Details
The Coalition on Reducing Bureaucracy in Clinical Trials, in which BioMed Alliance is an active member, just published a series of concrete and consensus-based recommendations for reducing administrative burdens in clinical trials. The recommendations reflect the needs and perspectives of investigators and patients, but are also taking into account the views of regulators, sponsors, ethics committees and other stakeholders to ensure the broadest possible uptake of the proposed the solutions.
The recommendations are gathered in four clusters: safety reporting, informed consent, regulatory guidelines and harmonisation of requirements accross the EU.
The Coalition Recommendations are available for reading on the website of the Coalition here.
Update November 2021
- Details
Read our new November Update to stay up-to-date on BioMed Alliance activities and EU policy developments. In this edition you will find more information on our General Assembly, upcoming leadership changes, the revised Statutes & Code of Conduct, our on session AI & Healthcare, a survey on regulatory affairs and the results of our IVDR Survey.
Join us for the BioMed Alliance policy session on Artificial Intelligence in healthcare
- Details
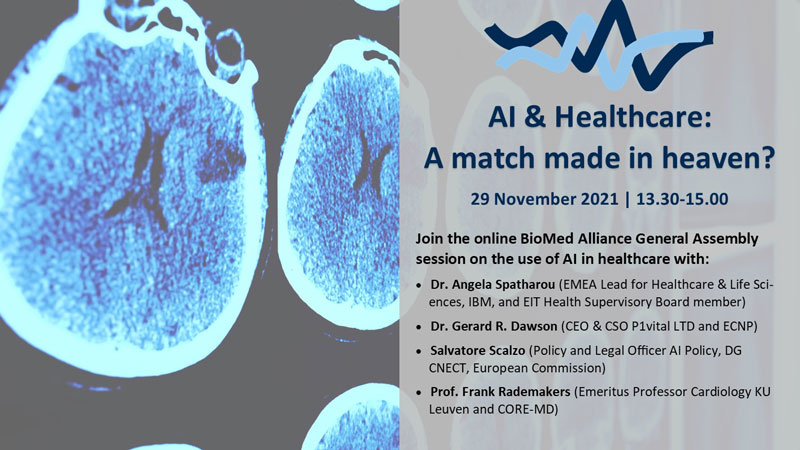
AI & Healthcare: a match made in heaven? The use of Artificial Intelligence in healthcare is significantly increasing, with promising new developments that are transforming healthcare. AI has enormous potential to enhance patient care and facilitate health research, but there are also challenges and risks ahead.
Join us on 29 November from 13.30-15.00 for an online discussion on the future of healthcare and the integration of AI in clinical practice and health research. At the occasion of the BioMed Alliance General Assembly, a series of interesting speakers will share their experience and knowledge on the use of AI & the regulatory framework that intends to facilitate the development and use of AI technologies, while mitigating risks. There will also be time for questions and reflection on what the future will look like, and how we can ensure that clinicians, healthcare professionals and patients are part of the transition.
Speakers:
- Dr. Angela Spatharou (EMEA Lead for Healthcare & Life Sciences, IBM, and EIT Health Supervisory Board member)
- Dr. Gerard R. Dawson (CEO & CSO P1vital LTD and ECNP)
- Salvatore Scalzo (Policy and Legal Officer AI Policy, DG CNECT, European Commission)
- Prof. Frank Rademakers (Emeritus Professor Cardiology KU Leuven and CORE-MD)
The session is open to all upon registration here. More information is available on the agenda.
Europe: Follower or leader in health research and innovation? BioMed Alliance organises discussion on Europe's ability to facilitate innovation at World Health Summit.
- Details

3 DAYS - 100 NATIONS - 300 SPEAKERS - 6,000 PARTICIPANTS - BERLIN & DIGITAL
The annual World Health Summit took place from 24-26 October and included a series of interesting sessions with keynote speakers like Commission President Ursula von der Leyen, Minister Jens Spahn, Commissioner Stella Kyriakides, Dr. Tedros Adhanom Ghebreyesus and many more.
The BioMed Alliance organised a session on Sunday 24 October at 11.00: Europe: Follower or Leader in Health Research and Innovation? The session was chaired by BioMed Alliance President Wilfried Ellmeier and featured prominent speakers including Dr. Özlem Türeci (Chief Medical Officer BioNTech), Jan-Philipp Beck (CEO EIT Health), Jan Geissler (CEO Patvocates), Prof. Karin Sipido (KU Leuven) and Robert Madelin (Chairman FIPRA). Discussions dove into the research and innovation landscape in Europe, persisting challenges and what Europe must do to position itself as a frontrunner in Biomedical and Translational research.
Update October 2021
- Details
Read our new October Update to stay up-to-date on BioMed Alliance activities and EU policy developments. In this edition you will find more information on our recent session at the World Health Summit, a new statement on HERA, the newly announced transitional provisions for the IVDR, the recent TEHDAS stakeholder forum, new health research funding calls and members' news.
New BioMed Alliance Statement on HERA: Will new agency live up to citizens’ expectations?
- Details
The European Commission has recently published its Communication on the new Health Emergency Response Authority (HERA) which is supposed to be established within the Commission and start operations early 2022. The BioMed Alliance welcomes the plans to establish HERA, but also raises certain concerns and needs for clarification in a new statement. In the document, medical societies highlight the need for better involvement of the scientific community, more extensive communication to stakeholders and the public and a good budgetary balance between HERA development and the realisation of other important health priorities.
Read the statement here

Update September
- Details
Read our new September Update to stay up-to-date on BioMed Alliance activities and EU policy developments. In this edition you will find more information on: our recent and upcoming events and meetings, the work of our IVDR Taskforce, the Commission’s plans for the new HERA, a European Parliament resolution on animal testing, information on interesting EMA and TEHDAS events and news from our members.
Join us to discuss health research at high level health events in the coming months!
- Details
The BioMed Alliance has been contributing to several high level events to facilitate discussions on research and innovation with leading opinion makers. On 28 August, President Wilfried Ellmeier led a session at the European Forum Alpbach focussing on 'Financing Research and Innovation in a European Health Union - NO CURE FOR LIFE'. In the coming weeks, we will also contribute to a session at the European Health Forum Gastein and organise a session at the world health summit.
European Health Forum Gastein
BioMed Alliance President-Elect Gunhild Waldemar will feature in the session 'the Heartbeat of Health Innovation' organised by EIT Health on 28 September at 11.00 during the European Health Forum Gastein. The session will focus on how networks & collective action can help us cope with crises and lead the way to a more resilient future.
More information is available here.
World Health Summit
On 24 October from 11.00-12.30 CET the BioMed Alliance is organising a session entitled 'Europe: Follower or Leader in Health Research and Innovation?' with prominent speakers. The session moderated by our president Wilfried Ellmeier features Dr. Özlem Türeci (BioNTech Chief Medical Officer), Prof. Karin Sipido (KU Leuven), Jan Geissler (Patvocates) and more to be announced soon. The focus of the discussion will be on how EU research can remain competitive and how EU health research initiatives can help work towards reaching important health objectives.
More information is available here.








